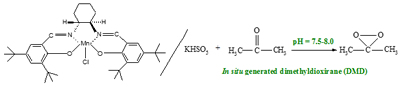
| Home | Academic and Research Staff |
| Equipment and services | |
| Publications | Research lines |
| Projects | |
| Contact and location | Thesis |
| Patents |
|
1. HETEROGENEOUS CATALYSIS
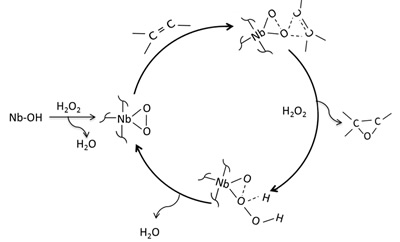
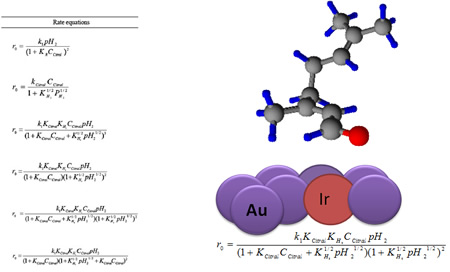
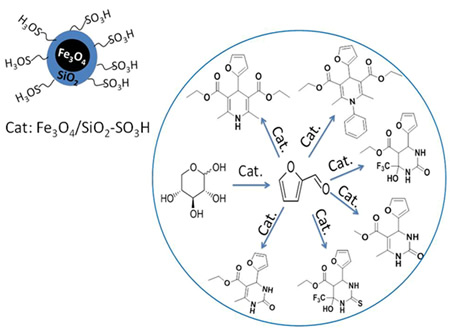
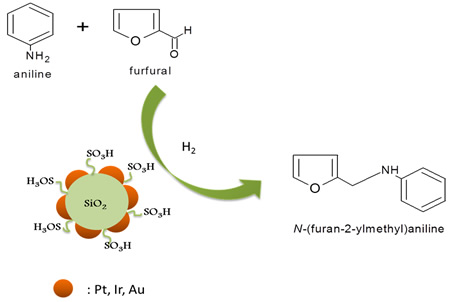
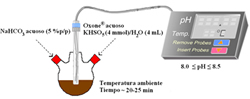
Dehydration of sugars to furfurals and its valorization via different reactions: Hydrolysis of lignocellulosic biomass can lead to the obtention of hexoses (glucose and fructose) and pentoses (xylose); then, the dehydration of these sugars to 5-hydroxymethylfurfural (HMF) and 2-furfural (FAL). Both HMF and FAL are building blocks widely applied to the next generation of biofuels, bio-based plastics, adhesive monomers, ecological adhesives, coating agents and fine chemical products. In our research group different reactions for the transformation of furfurals have been employed, some examples of these reactions are presented as follows: a. Multicomponent reactions (MCR): We used the versatility of the furfural molecule as building block in the Biginelli and Hantzsch-type multicomponent reactions to obtain different polyfunctional nitrogenated heterocycles using green protocols, including a solvent-free reaction and a recyclable solid acid with magnetic properties (Fe3O4/SiO2-SO3H), this solid is easily separable from the reaction medium. b. Reductive Amination: We prepare bifunctional catalysts, which can favor the reductive amination of furfural with amino or nitro compounds. This reaction should proceeds slowly without the acidic sites; however the acid sites should protonate carbonyl group making the carbonylic carbon a better electrophile and activates it more easily towards nucleophilic attacks in the first step which favors the hydrogenation of the imine and consequently the desired amine formation. c. Selective Hydrogenation: Our researchers have studied the liquid phase furfural hydrogenation using Ir catalysts. Ir catalysts displayed a high activity and selectivity in the hydrogenation of C=O group of α, β-unsaturated aldehydes. The SMSI effect has been broadly studied for explain the catalytic behavior of this type of catalysts and the selectivity to unsaturated alcohol. d. Oxidation of HMF: Recently, we start the study the oxidation of HMF with bimetallic catalysts (Au-Me) for obtain 2,5-furandicarboxylic acid (FDCA). Solids developed a. Acid solid catalysts with magnetic properties: To address the issue of reuse, sulfonated solid catalysts and heteropolyacids micellar with magnetic properties have been used. The paramagnetic properties of the solids allow easy separation of the catalyst in the reaction mixture using an external magnetic field. Aggregation of the particles can be avoided by the use of stabilizers or by the formation of a passive layer of silica or other oxide on the surfaces of nanoparticles (NPs) of iron oxide. These solids have been employed in multicomponent reactions. b. Au-Me (Me: Ir, Pt) catalysts. Our group has studied the addition of gold over metallic catalysts (group 8-10) for increases the reduction process in distinct α, β-unsaturated carbonyl aldehydes. The possibility of that the addition of Au may modify the electronic properties of Me (group 8-10). We demonstrated that in Ir-Au/TiO2 catalysts there an electronic charge transfer from the iridium atoms to the gold.
2. CATALYSIS INDUCED BY METALS AND METALLIC OXIDES
a. Catalysis by metals
b. Acid catalysis
c. Bifunctional catalysis
3. ENANTIOSELECTIVE CATALYSIS
4.HETEROGENEOUS PHOTOCATALYSIS
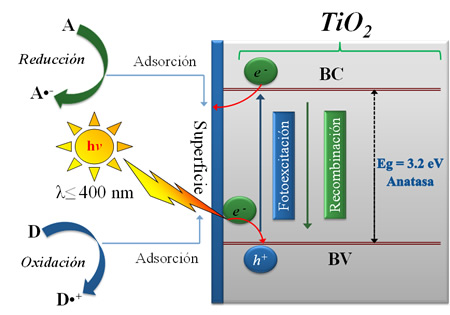
In the last years, heterogeneous photocatalysis has appeared as an efficient and eco-friendly remediation technology; thus, a wide variety of VOCs in gas phase are decomposed on the UV-irradiated TiO2 surface under ambient conditions without any chemical additives. Currently, photocatalysis based on titanium dioxide (TiO2) has also demonstrated to be a good alternative in the treatment wastewater polluted with microorganisms and organic compounds coming from different industrial and domestic activities. This less expensive technology leads to recover water sources by using artificial or direct solar light, which are available anywhere in the world. The Grupo de Catálisis de la Universidad Pedagógica y Tecnológica de Colombia has focused its efforts on the development of new materials and alternatives for the improvement of the photocatalytic processes and reaction systems used in environmental remediation.
|
||
|
Información actualizada: 24 de mayo de 2017 |